There are some stories that, you know, just stick with you, sending a real chill down your spine. One such tale, a truly unsettling one from history, involves a series of very strange disappearances and a most unusual way of making bodies vanish. This particular story is often called the "acid bath murder," and it really does bring up some very uncomfortable thoughts about human nature and, too it's almost, the limits of what some people might do.
This rather grim part of history, actually, centers on a method that used powerful chemicals to make human remains disappear. It's a case that, in some respects, made people question a lot about how crimes could be covered up, and it shows a dark side of using everyday substances in a way that was never intended. The person behind these awful acts, a man named John George Haigh, became infamous for his cold, calculating approach to doing away with those he had wronged.
So, as we look into this disturbing historical event, we'll also take a little bit of time to talk about the very substances that played such a key role. What exactly is an acid? How does it work? And how could such a thing be used in a manner so shocking? We'll explore the basic properties of these liquids, you know, the kind of information that helps us grasp the unsettling details of the "acid bath murder" case, and what happened when a person tried to erase all traces of their deeds.
Table of Contents
- What is an Acid, Anyway?
- The Acid Bath Murder - A Shocking Crime
- Who Was Behind the Acid Bath Murder?
- John George Haigh - A Disturbing Figure
- How Did the Acid Bath Murder Happen?
- The Gruesome Method of the Acid Bath Murder
- What Did the Acid Do in the Acid Bath Murder?
- The Science of Dissolution in the Acid Bath Murder
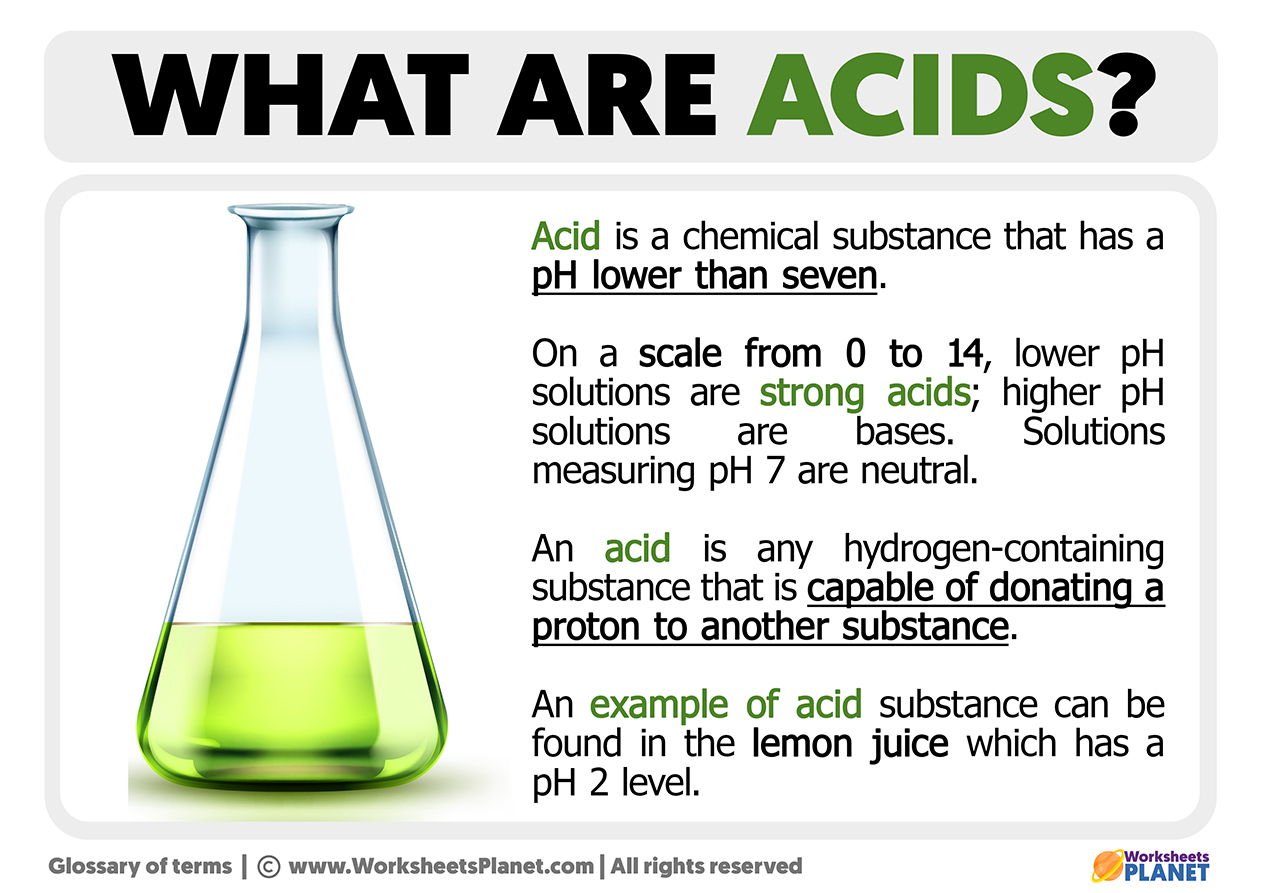
What is an Acid, Anyway?
Before we get too deep into the darker parts of this story, it might be helpful to, you know, just get a basic idea of what an acid really is. When we talk about these kinds of liquids, we're usually referring to something that has some very particular characteristics. For one thing, as a matter of fact, if you were to taste an acid that's been mixed with water, it would have a very sharp, puckery flavor. Think about how a lemon makes your mouth feel; that sort of intense, sour sensation is one of the classic signs of an acid.
Another way to tell if something is an acid, as I was saying, involves a special kind of paper called litmus paper. If you have a piece of blue litmus paper and you put it into an acidic liquid, you would see it change color. That blue paper would turn a bright red. This color change is a pretty clear signal that you are dealing with a substance that has acidic properties. It’s a simple test, really, but it tells you a lot about the chemical makeup of the liquid you're looking at, giving you a quick visual cue.
Acids also, you know, have a tendency to react with certain types of metals. When this happens, a gas called hydrogen can be given off. You might see bubbles forming, which is a sign of this reaction taking place. This ability to react with metals is a very important feature of acids, and it shows just how active these substances can be. It's a chemical interaction that can be quite powerful, basically, causing visible changes to the materials involved.
Then there's the way acids react with what we call "bases." When an acid and a base get together, they often form something new, which chemists call a "salt," and sometimes water too. This is a kind of balancing act, you know, where the acid's strong properties are calmed down by the base. It’s a very common type of chemical reaction, and it's something that happens all the time in different parts of the world, whether we notice it or not. This process is, you know, often referred to as a neutralization reaction.
In the world of chemistry, there are, you know, a couple of main ways that people think about what makes an acid an acid. One idea, which was first described by a person named Arrhenius back in 1884, says that an acid is a chemical thing that gives away hydrogen parts, or protons. It's like, you know, it has extra hydrogen bits it wants to share. Another way to look at it is that an acid can be something that accepts a pair of tiny electron pieces. So, really, it's about how these substances interact at a very small, atomic level, either giving away or taking in certain particles. These definitions, you know, help scientists categorize and understand the behavior of different acidic substances, giving them a framework to work with.
The Acid Bath Murder - A Shocking Crime
Now, with a little bit of a clearer picture of what acids are, we can, you know, start to think about how such powerful substances could be used in a truly awful way. The "acid bath murder" is a name given to a series of crimes that happened in England during the 1940s. It’s a very grim story that involves a person who used these corrosive liquids to try and get rid of all traces of the people he had killed. The sheer coldness of the idea, you know, to dissolve a human body, made this case stand out in a very disturbing way to the public.
The details of this particular crime are, as a matter of fact, quite chilling. It wasn't just one instance, but a pattern of behavior where the perpetrator believed he could make his victims disappear without a trace. The use of acid was central to his plan, a way to ensure that no bodies would ever be found, which he thought would mean no proof of his wrongdoing. This method, you know, was something that shocked the nation and, too it's almost, made people wonder about the kind of person who could even think of such a thing, let alone carry it out.
Who Was Behind the Acid Bath Murder?
The person at the center of the "acid bath murder" case was, you know, a man named John George Haigh. He was, in some respects, a rather unusual figure, someone who seemed to live a life that, on the surface, might have appeared quite ordinary to some. However, underneath that veneer, there was a very dark and calculating mind at work. His actions would eventually reveal a person capable of extreme cruelty and a chilling disregard for human life. So, who was this man who came to be known for such a horrific method of disposal?
John George Haigh was, you know, a man who had a history of getting into trouble, though perhaps not always with the level of violence that would later define him. He had been involved in various schemes that were, basically, dishonest, and had spent time in prison before. These experiences, it seems, did not deter him from a path of wrongdoing. In fact, they might have, in a way, made him even more determined to find ways to commit crimes without getting caught, leading him to think about very extreme measures like the use of strong acids. He was, basically, a person who was always looking for an angle, a way to benefit from others, regardless of the cost to them.
John George Haigh - A Disturbing Figure
Here are some basic details about John George Haigh, the person connected to the "acid bath murder" case:
| Full Name | John George Haigh |
| Born | July 24, 1909 |
| Birthplace | Stamford, Lincolnshire, England |
| Died | August 10, 1949 |
| Cause of Death | Execution by hanging |
| Known For | Serial killings, dissolving victims' bodies in acid |
| Nickname | The Acid Bath Murderer |
Haigh's background, you know, didn't necessarily scream "future murderer" to those around him at first. He grew up in a very strict religious household, which, in some respects, might have given him a rather warped sense of morality later on. He was, for example, a choirboy in his youth, which is quite a contrast to the person he became. His early adult life was marked by a series of failed business attempts and, you know, stints in prison for fraud. These experiences, it seems, shaped his outlook, making him believe that he could outsmart the law and get away with almost anything, especially if he could make the evidence disappear completely.
He was, in a way, a chameleon, able to present himself as a respectable person to his victims, gaining their trust before, you know, carrying out his terrible plans. This ability to blend in and appear harmless was, basically, a key part of his method. He would target people who were, perhaps, a little bit isolated or who had money that he could take. His calm demeanor, even after committing such awful acts, was something that, you know, really disturbed those who encountered him. It showed a person who had, basically, lost all sense of normal human feeling, and was driven by a desire for personal gain, no matter what.
How Did the Acid Bath Murder Happen?
The method John George Haigh used in the "acid bath murder" cases was, you know, truly horrifying and, too it's almost, unique in its brutality. His plan was simple in its goal: to leave no trace of his victims. To achieve this, he turned to the very powerful properties of acids, substances we talked about earlier that can, you know, react with things and break them down. He acquired large drums and, basically, filled them with strong, corrosive acids, creating a kind of macabre vat for his terrible purpose.
After he had taken a person's life, Haigh would, you know, place the body into one of these large containers. He would then pour in the strong acid. The idea was that the acid would, over time, eat away at the human remains, dissolving them into a sludge that could then be poured away. This process was, basically, meant to ensure that no recognizable parts of the body would ever be found, which he believed would make it impossible for the authorities to prove that a murder had even happened. It was a very cold and calculated approach to getting rid of evidence, showing a mind that had thought through every gruesome step.
He would, you know, often check on the dissolving process, sometimes adding more acid if he felt it was needed to speed things along. This was not a quick process, by the way; it took time for the acid to do its work. The goal was to reduce the body to, basically, nothing more than a thick, brownish liquid and some bone fragments that were too hard for the acid to completely break down. This leftover material, you know, he would then dispose of in various places, like drains or open ground, further trying to erase any sign of his crimes. It was a horrifying example of how, in some respects, a person could use scientific knowledge for the most terrible ends.
The Gruesome Method of the Acid Bath Murder
The exact chemicals John George Haigh used for the "acid bath murder" were, basically, very strong industrial acids, often sulfuric acid, which is known for its ability to break down organic matter. He would get these acids, you know, from various sources, sometimes claiming he needed them for business purposes. The sheer volume of acid he used was, in a way, quite significant, as he needed enough to fill large containers that could hold a human body. This was, as a matter of fact, a key part of his method, as weaker acids would not have been effective enough for his chilling plan.
The process involved, you know, a lot of careful handling of these dangerous liquids. Acids, as we talked about, can react with many things, and they can cause a lot of harm if not handled properly. Haigh, it seems, was very aware of the risks, but he was also determined to carry out his plan. He would often conduct these gruesome activities in a secluded workshop or, you know, in the basement of a property, trying to keep his actions hidden from anyone who might notice the strange smells or sounds that would come from such a process. It was a very isolated and secretive operation, which, in some respects, made it even more disturbing.
The idea of using acid to make a body disappear was, basically, something Haigh believed would make him immune to being caught. He thought that if there was no body, there could be no murder conviction. This belief, you know, was a driving force behind his method. He was, in a way, trying to exploit a loophole in the justice system, thinking he was clever enough to get away with his terrible deeds. The gruesome reality of the "acid bath murder" method, however, eventually led to his capture, as even acid cannot completely erase all traces of a human being.
What Did the Acid Do in the Acid Bath Murder?
So, what exactly did the acid do to the bodies in the "acid bath murder" cases? When a strong acid comes into contact with organic material, like human tissue, it starts a very powerful chemical reaction. You know, we talked about how acids can donate hydrogen ions or accept electron pairs. This activity is what makes them so reactive. In simple terms, these acids work by breaking apart the very complex structures that make up a living body. They attack the proteins, fats, and other components, turning them into simpler substances that can dissolve in the liquid.
The acids used by Haigh were, basically, very good at this kind of breakdown. They would, you know, eat away at the soft tissues, turning them into a kind of thick, dark liquid. This process is, in a way, similar to how a very strong cleaner might break down grease, but on a much more intense and destructive scale. The acid would work on the muscles, organs, and skin, reducing them over time. It's a very stark reminder of the power of these chemical reactions and, too it's almost, how they can transform matter from one state to another.
However, even the strongest acids have their limits. While they are very effective at breaking down soft tissues, there are some parts of the human body that are much harder for them to affect. Things like bones and teeth, which are made of mineral compounds, are much more resistant to acid. So, while the soft parts would dissolve, some fragments of bone or dental work would often remain. This fact, you know, was actually very important in the eventual investigation of the "acid bath murder" cases, as these small, resistant pieces of evidence were, basically, what ultimately helped to prove what had happened.
The Science of Dissolution in the Acid Bath Murder
The chemical process of dissolution in the "acid bath murder" was, basically, a very aggressive form of what we call hydrolysis, where water molecules, often with the help of the acid, break down other molecules. When we talked about acids reacting with bases to form salts, you know, it's a bit like that, but in this case, the "base" is the organic material of the body itself. The acid acts as a powerful agent, stripping away hydrogen ions or, you know, accepting electrons from the complex biological molecules, causing them to fall apart.
The effectiveness of this dissolution process depends, in some respects, on several things. The strength of the acid is, of course, very important; a stronger acid will work much faster and more completely. The temperature also plays a role; warmer conditions can speed up chemical reactions. And, you know, the amount of acid used compared to the amount of material to be dissolved also matters a lot. Haigh, it seems, understood these factors, at least in a practical sense, as he used large quantities of very strong acids and, you know, sometimes even heated the mixture to try and make the process more efficient.
This horrifying use of chemistry in the "acid bath murder" cases serves as a very stark reminder that scientific principles, which are, basically, neutral in themselves, can be twisted for the most terrible purposes. The ability of acids to break down organic matter is a known chemical property, but its application in this context was, you know, truly shocking. It highlights how a basic scientific idea, when applied with very dark intentions, can lead to unspeakable acts, leaving a lasting mark on history and, too it's almost, our collective memory of crime.